Articles from Medable Inc.

Medable Inc., a leading provider of clinical development technology, announced that its eConsent and eCOA solutions have been approved for use in two clinical studies in eight countries outside of the European Union by France’s Commission Nationale de l’Informatique et des Libertés (CNIL). This milestone makes Medable uniquely capable of operating as a data processor outside of the CNIL MR-001 Reference Methodology requirements for a France-based clinical trial sponsor. The achievement underscores Medable’s commitment to expanding access and providing secure, compliant, and scalable digital solutions for global clinical research.
By Medable Inc. · Via Business Wire · March 4, 2025

Please replace the release dated Jan. 28, 2025 with the following corrected version due to multiple revisions.
By Medable Inc. · Via Business Wire · January 29, 2025

Medable Inc., a leading provider of clinical development technology, today announced 80% revenue growth in 2024 across enterprise customers adopting a SaaS model for portfolio-level electronic clinical outcomes assessment (eCOA) technology investments compared to study-by-study contracts. The striking increase suggests rising industry confidence in the use of digital technology in clinical trials and a strategy shift from tentative, careful adoption to wide scale implementation of eCOA solutions across therapeutic areas, portfolios, regions, and even across the enterprise.
By Medable Inc. · Via Business Wire · January 28, 2025

Medable Inc., a leading provider of clinical development technology, today announced Medable AI – generative AI capabilities that help sponsors and clinical research organizations (CROs) build digital and decentralized trials faster with complete visibility and control over technology setup. Medable is the first to incorporate generative AI in the study build process, ultimately driving the industry to a breakthrough, one-day study startup.
By Medable Inc. · Via Business Wire · November 12, 2024

Medable Inc., a leading provider of clinical development technology, today announced a collaboration with Google Cloud to bring Medable’s Digital and Decentralized Clinical Trial Platform to Google Cloud Marketplace. Customers will benefit from cutting-edge Google Cloud infrastructure and AI capabilities, seamlessly integrated with Medable’s platform, including the newly launched Studio, a unique SaaS solution that provides pharmaceutical companies control and transparency in clinical trial design, deployment, and management. Medable’s strategic collaboration with Google Cloud allows life sciences companies to accelerate clinical development while leveraging infrastructure and financial benefits.
By Medable Inc. · Via Business Wire · October 30, 2024

Medable Inc., the leading technology platform for clinical trials, today announced it was named Trailblazer in Everest Group’s inaugural “Clinical Trial Patient Engagement Products Assessment.” Medable was selected out of 44 organizations and one of the only companies to receive a perfect score in all categories for its holistic focus on patient engagement in clinical trials, as reflected in its Patient Caregiver Network (PCN) – the industry’s first, largest, and most diverse patient advocacy ecosystem and cornerstone to Medable R&D.
By Medable Inc. · Via Business Wire · October 22, 2024

Medable Inc., the leading technology platform for clinical trials, today announced Medable Studio, an all-in-one application for configuring, translating, validating, and launching eCOA Plus (eCOA, eConsent, Televisit, Sensors) into clinical trials. Studio is a no-code suite that simplifies the complex eCOA launch process, giving biopharmaceutical companies greater control and transparency for faster study go-live and earlier patient enrollment. Customers choose between self-service, full-service, or a combination of options, all providing increased time and cost efficiency.
By Medable Inc. · Via Business Wire · August 14, 2024

Medable Inc., the leading technology provider for modern clinical trials, today announced that its evidence generation platform was awarded “Best Digital Health Solution” from a field of 17 nominees at the Prix Galien UK Forum in London. This marks the second time Medable has won the Best Digital Health Solution award, first taking home the honor in 2023 at the Prix Galien US ceremony.
By Medable Inc. · Via Business Wire · June 13, 2024

Medable Inc., the leading technology provider for modern clinical trials, today announced that the Partnership for Advancing Clinical Trials (PACT) consortium in conjunction with the Tufts Center for the Study of Drug Development (CSDD) has produced compelling results from a new study. In total, 13 clinical trial sponsors and contract research organizations provided robust data on 60 clinical trials that deployed decentralized solutions and found that actual timelines beat planned timelines from first site activated to first patient enrolled, and from first patient to last patient enrolled.
By Medable Inc. · Via Business Wire · June 11, 2024

Medable Inc., the leading evidence-generation platform provider for modern clinical trials, today announced that Medable Co-founder and CEO Michelle Longmire will present at the Jefferies Global Healthcare Conference. The live presentation will take place on Thursday, June 6, 2024, at 3:30p.m. ET in New York City, New York.
By Medable Inc. · Via Business Wire · May 30, 2024

Medable Inc., the leading technology provider for modern clinical trials, is partnering with industry leader Masimo (NASDAQMASI) to bring best-in-class medical-grade wearable devices to clinical research. Masimo’s award-winning pulse oximeter technology has been widely trusted by clinicians for over 25 years to monitor more than 200 million patients each year and is now enabling more patients to participate in clinical trials without the burdens of ongoing trips to the trial site for routine testing.
By Medable Inc. · Via Business Wire · May 29, 2024

Medable Inc., the leading technology provider for modern clinical trials, achieved an impressive 75% adoption of its Total Consent solution deployed in elderly patient population in U.S. Renal Care’s (USRC) investigator-initiated trial. The Phase IV trial, funded by a top-10 global pharmaceutical company, had a narrow recruitment window to enroll patients suffering from Anemia in End Stage Kidney Disease (ESKD) across more than 40 dialysis centers, involving 476 site users.
By Medable Inc. · Via Business Wire · April 16, 2024

Medable Inc., the leading technology provider for modern clinical trials, announces results from a new research project with Duke University Department of Population Health Sciences’ Bioethics and Stakeholder (BASE) Lab on the acceptability of enhanced electronic informed consent (eIC) in clinical trials. The pilot study* compared participant comprehension and usability, satisfaction, and preference of enhanced eIC versus text-only eIC, specifically exploring the use of interactive videos, graphics, calendars, and other tools to augment text in an eIC form.
By Medable Inc. · Via Business Wire · January 31, 2024

Medable Inc., the leading technology provider for modern clinical trials, announces new intelligent automation technology applied across its clinical trials platform to cut standard trial build timelines by at least half. Early application of the technology in electronic clinical outcomes assessment (eCOA) deployments – a major delay to study startup industry-wide – is groundbreaking. By automating laborious, manual tasks such as testing, Medable saves substantial time and removes eCOA from the critical path to trial go-live.
By Medable Inc. · Via Business Wire · January 8, 2024

Medable Inc., the leading technology provider for modern clinical trials, today announced that Medable CEO and Co-Founder Michelle Longmire will be presenting a corporate update at the 42nd Annual J.P. Morgan Healthcare Conference in San Francisco. The presentation will take place on Wednesday, January 10, 2024, at 7:30 AM Pacific Time (10:30 AM Eastern Time).
By Medable Inc. · Via Business Wire · January 3, 2024

Medable Inc., the leading technology provider for modern clinical trials, today announced that its evidence generation platform was awarded “Best Digital Health Solution” from a field of 24 nominees at the Prix Galien USA Forum in New York City. Worldwide, the Prix Galien Award is regarded as the equivalent of the Nobel Prize for the life science industry. Winners from this year’s cohort include respected companies such as Bristol Myers Squibb, Eli Lily and Company, Novo Nordisk Inc., Boehringer Ingelheim and more.
By Medable Inc. · Via Business Wire · October 30, 2023

Medable Inc., the leading technology provider for modern clinical trials, announces an enterprise partnership with Pluto Health, a smart care coordination services company. The strategic partnership brings together two best-in-class solutions – Pluto Health’s health coordination platform and Medable’s digitally enabled clinical trials platform – to accelerate clinical development, increase access to clinical research, and improve data quality. Notably, the partnership will revolutionize the patient experience by providing patients with greater control and the ability to share their health data directly into clinical trials through a seamless integration.
By Medable Inc. · Via Business Wire · October 3, 2023

Medable Inc., the leading technology provider for patient-centric clinical trials, today announced that it ranked in the top 8 percent (#398) and was one of the top 50 software companies on the 2023 Inc. 5000, its annual list of the fastest-growing private companies in America. Medable’s ranking was fueled by its strong three-year growth rate of 1,453% from 2019 through 2022.
By Medable Inc. · Via Business Wire · August 17, 2023
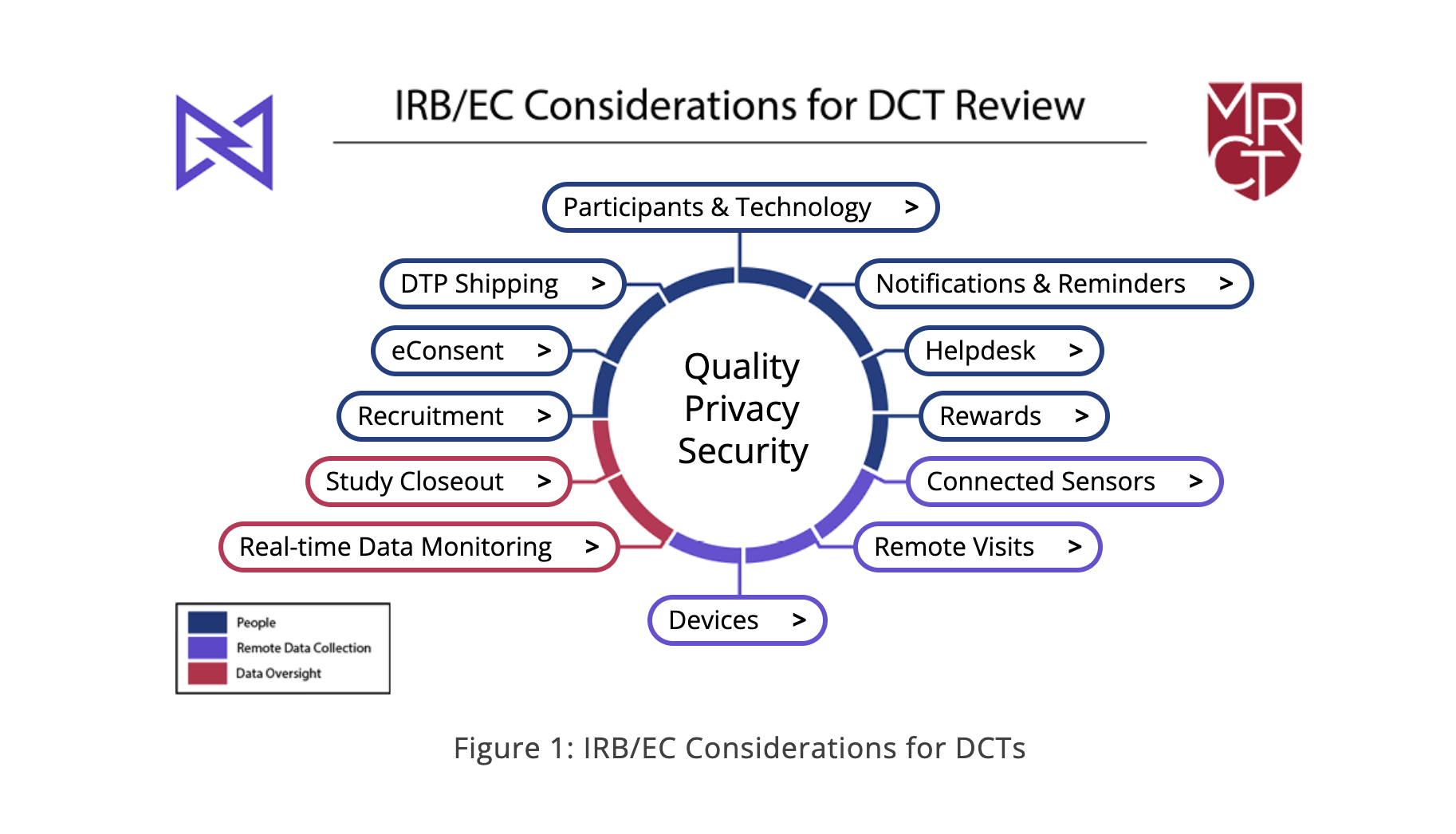
Medable Inc., the leading technology provider for patient-centric clinical trials, and the Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard (MRCT Center) announce a comprehensive toolkit for Institutional Review Boards (IRBs)/Ethics Committees (ECs) to standardize decentralized clinical trial (DCT) ethics review. The first-of-its-kind toolkit provides a common framework, tools, and best practices for uniform ethical review and approval and provides a roadmap for the ethical conduct of DCTs. Ultimately, such standardization will simplify, streamline, and speed the IRB/EC process – a key step towards more efficient, patient-centered research execution.
By Medable Inc. · Via Business Wire · June 20, 2023

Medable Inc., the industry-leading technology platform for patient-centered clinical trials, today announced a partnership with non-profit Every Cure, which officially launched in September in partnership with the Clinton Global Initiative to scale up an innovative “drug repurposing” research approach to identify treatments for rare diseases.
By Medable Inc. · Via Business Wire · January 4, 2023

Medable Inc., the industry-leading technology platform for patient-centered clinical trials, today announced that it was named a Leader in the “Decentralized Clinical Trial Products PEAK Matrix® Assessment 2022” by Everest Group for the second consecutive year. The report evaluates decentralized clinical trial (DCT) products from 24 companies based on vision, capability and market impact. Medable rated highest in the Leaders quadrant with top scores for vision, capability and market impact.
By Medable Inc. · Via Business Wire · November 11, 2022

Medable Inc., the industry-leading technology platform for patient-centered clinical trials, today announced the availability of its Total Consent offering, a fully enabled SaaS electronic consent management solution compatible with every clinical trial, in more than 120 locales around the globe. With this launch, Medable’s Total Consent offering is at the forefront of optimizing the informed consent process for potential trial participants by improving patient accessibility, knowledge, and experience, in line with the release of the proposed FDA rule, Protection of Human Subjects and Institutional Review Boards.
By Medable Inc. · Via Business Wire · November 3, 2022

Medable Inc., the industry-leading technology platform for patient-centered clinical trials, today announced a new patient-first oncology offering that enables life sciences companies to improve patient access, enrollment, and retention in cancer trials. Medable’s new offering is an end-to-end suite that includes pre-built and validated DCT applications such as Total Consent and Televisit, an extensive eCOA oncology library, and protocol design consulting – with a single point of entry for patients, sites and sponsors.
By Medable Inc. · Via Business Wire · October 26, 2022

Medable Inc., the leading software provider for patient-centered clinical trials, today announced that it has entered a four-year enterprise contract with GSK to enable decentralized clinical trials (DCTs) across their portfolio using the company’s industry-leading DCT platform. Medable was selected by GSK to accelerate the delivery of new medicines and enable their clinical trials to be more inclusive and representative of all patient populations.
By Medable Inc. · Via Business Wire · September 7, 2022

Medable Inc., the leading SaaS platform for patient-centered clinical research, today announced new software solutions that enable life sciences companies to expedite critical vaccine development and quickly respond to outbreaks like COVID-19, monkeypox and new flu variants. The solutions include pre-configured modules for vaccine clinical trials that increase operational efficiency based on industry best practices, while cutting deployment times from more than 12 weeks on average to as little as five weeks.
By Medable Inc. · Via Business Wire · August 30, 2022

Medable Inc., the leading SaaS platform provider for patient-centered clinical trials, today announced a new partnership with Withings Health Solutions, the business-to-business division of Withings, one of the global leaders in at-home connected health. Withings’ devices will seamlessly connect to Medable’s decentralized clinical trial platform, reducing the burden on sponsors and sites while empowering patients to participate in trials from home.
By Medable Inc. · Via Business Wire · July 21, 2022

Medable Inc., the leading SaaS platform for patient-centered clinical trials, today announced the appointment of enterprise software veteran and Dialpad CFO Mike Kourey to its board of directors. Kourey, who brings unparalleled expertise in scaling both public and private enterprise software companies, will help guide Medable’s continued rise as a leader in the health technology industry.
By Medable Inc. · Via Business Wire · April 7, 2022

Medable Inc., the leading software provider for patient-centered clinical trials, today launched the Medable Partner Network – uniting a diverse ecosystem of technology, service, data, site, and direct-to-patient partners that work together to accelerate deployment of decentralized clinical trials. The network includes new partnerships with Advanced Clinical, Cognizant and CVS, and expanded relationships with Datavant, Oracle, Parexel, PPD, part of Thermo Fisher Scientific, and Syneos Health. These partners share in Medable’s mission to get more effective therapies to patients faster.
By Medable Inc. · Via Business Wire · February 8, 2022

Medable Inc. and CVS Health (NYSECVS) today announced a collaboration to expand clinical trial access and engagement for patients at select MinuteClinic™ locations via Medable’s clinical trials software platform. CVS Health Clinical Trial Services™ will manage the relationship.
By Medable Inc. · Via Business Wire · February 7, 2022

Medable Inc., the leading software provider for patient-centered clinical trials, today announced that it has acquired LEO Innovation Lab’s Omhu A/S, a Denmark-based digital health tech company. Omhu was launched in 2020 to develop new ways of supporting patients and doctors when diagnosing, managing, and treating skin conditions. As part of the agreement with LEO Pharma A/S (owner of LEO Innovation Lab), Medable will take over the Omhu mobile applications and a talented team of 38 people with significant experience in digital health and artificial intelligence.
By Medable Inc. · Via Business Wire · February 2, 2022

Medable Inc., the leading cloud platform for patient-centered drug development, today announced results from a Tufts CSDD analysis which shows that, on average, decentralized clinical trials (DCTs) are associated with reduced clinical trial timelines and can achieve net financial benefits ranging from five to 14 times for Phase II and Phase III trials, respectively. The findings are based on financial modeling and analysis of trial data from the Tufts Center for the Study of Drug Development (CSDD), Tufts University School of Medicine, and more than 150 clinical trials enabled by Medable software.
By Medable Inc. · Via Business Wire · January 13, 2022

Medable Inc. and Vault Health, Inc. today announced that the two fast-growing digital health leaders are teaming up to integrate Vault’s expertise in diagnostics, logistics and remote care services with Medable’s end-to-end software platform for decentralized clinical trials. The partnership builds on a shared commitment to bring trials closer to patients via remote monitoring and high-touch patient services – helping make clinical trials more inclusive and accessible, and ultimately enabling new therapies to come to market faster.
By Medable Inc. · Via Business Wire · December 8, 2021

Medable Inc., the leading cloud platform for patient-centered clinical research, today announced $304 million in Series D funding to accelerate global adoption of digital and decentralized clinical trials, enabling ubiquitous access to the latest treatments for every body and every biology. The funding round was co-led by new investors Blackstone Growth and Tiger Global and existing investor GSR Ventures. The round also includes follow-on investment from existing investors Sapphire Ventures and WTI.
By Medable Inc. · Via Business Wire · October 26, 2021

Medable Inc., the leading cloud platform for patient-centered clinical research, today announced the hiring of Orlando Baeza as the company’s first chief marketing officer (CMO). Baeza is an experienced technology and consumer marketing leader who was most recently CMO for technology startups Kajabi and Pollen, and previously led innovative marketing initiatives for Nike, Adidas, Paramount, Activision and BuzzFeed. Baeza has been recognized as a trendsetting marketer via Forbes “30 under 30” and Brand Innovators “40 under 40.”
By Medable Inc. · Via Business Wire · October 12, 2021

Medable Inc., the leading cloud platform for patient-centered drug development, today announced its Site Network Council (SNC), a select group of clinicians and research leaders who will serve as influential voices in Medable product and services development. SNC members represent diverse clinical sites and provide real-world experience and insights to inform Medable technical and strategic direction. They offer a transparent window into modern research environments to help Medable create decentralized clinical trial workflows that meet sites’ changing needs and enable greater participation in DCTs.
By Medable Inc. · Via Business Wire · September 29, 2021

Medable Inc., the leading cloud platform for patient-centered drug development, today announced the hiring of Dr. MaryAnne Rizk as Chief Strategy Officer. Rizk is a veteran leader with more than 20 years of experience managing partnerships and ecosystems to accelerate development across technology (Oracle, Medidata), pharmaceutical (Merck), and clinical research organizations (IQVIA).
By Medable Inc. · Via Business Wire · September 7, 2021

Medable Inc., the leading cloud platform for patient-centered drug development, today announced that it was named a Leader in the “Decentralized Clinical Trial Products PEAK Matrix®” assessment by Everest Group. The new report, issued last Friday, evaluates decentralized trial products from 15 companies based on vision, capability and market impact. Medable ranked highest in the Leaders quadrant with top scores for both vision and capability as well as market impact.
By Medable Inc. · Via Business Wire · July 20, 2021

Medable Inc., the leading cloud platform for patient-centered drug development, today announced the first stage of its European expansion plans with a new EMEA headquarters in Dublin. The regional headquarters will serve as a hub for expanded sales, customer success and software development in Europe, enabling Medable to continue broad adoption of digital clinical trials at global scale.
By Medable Inc. · Via Business Wire · July 14, 2021

Medable Inc., the leading software platform for patient-centered drug development, today announced an industry-first digital certification program that provides life sciences companies with specialized tools, knowledge sharing and skills development to rapidly scale their decentralized and hybrid trial strategies.
By Medable Inc. · Via Business Wire · June 24, 2021

Medable Inc., the leading cloud platform for patient-centered drug development, today announced the hiring of Sanskriti Thakur as chief growth officer. Thakur most recently served as global life sciences research lead for Accenture, responsible for market-shaping strategy and research in therapeutics, digital health, and business model innovation. Notably, she was responsible for perspectives in new science, COVID-19 responses, and clinical trial diversity.
By Medable Inc. · Via Business Wire · June 17, 2021

Medable Inc. today announced $78 million in new funding to fuel advances in the delivery of digital and decentralized clinical trials, accelerating the industry’s shift to patient-centered drug development. The funding was led by Sapphire Ventures, along with new investor Obvious Ventures and follow-on investment from existing investors GSR Ventures, PPD, Inc. (Nasdaq:PPD) and Streamlined Ventures.
By Medable Inc. · Via Business Wire · April 15, 2021
